PICS (Pharmaceutical Inspection Convention (PIC) and the Pharmaceutical Inspection Co-operation Scheme)
The Pharmaceutical Inspection Co-operation Scheme (PIC/S) is a non-binding, informal co-operative arrangement between Regulatory Authorities in the field of Good Manufacturing Practice (GMP) of medicinal products for human or veterinary use. It is open to any Authority having a comparable GMP inspection system. PIC/S presently comprises 54 Participating Authorities coming from all over the world (Europe, Africa, America, Asia and Australasia).
PIC/S aims at harmonising inspection procedures worldwide by developing common standards in the field of GMP and by providing training opportunities to inspectors. It also aims at facilitating co-operation and networking between competent authorities, regional and international organisations, thus increasing mutual confidence. This is reflected in PIC/S’ mission which is to lead the international development, implementation and maintenance of harmonised GMP standards and quality systems of inspectorates in the field of medicinal products.
This is to be achieved by developing and promoting harmonised GMP standards and guidance documents; training competent authorities, in particular inspectors; assessing (and reassessing) inspectorates and facilitating the co-operation and networking for competent authorities and international organisations.
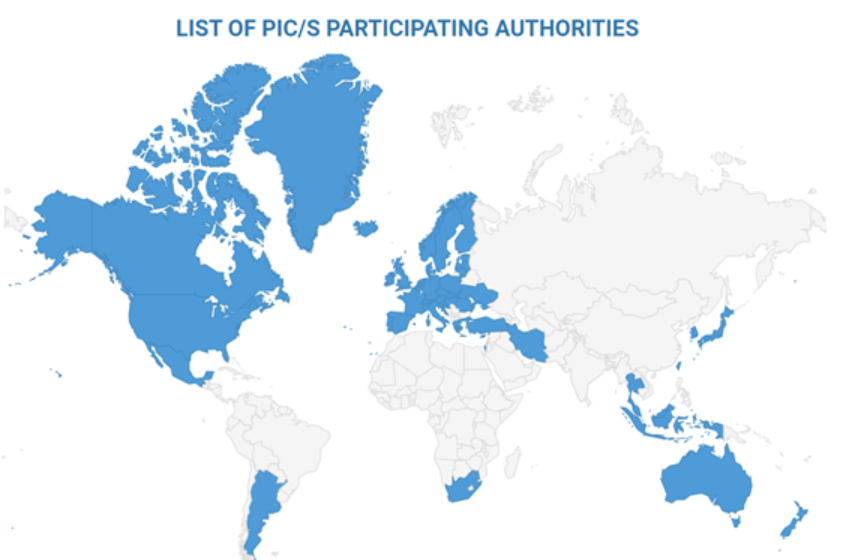
1. Argentina
National Institute of Drugs
Instituto Nacional de Medicamentos (INAME)
2. Australia
Therapeutic Goods Administration (TGA)
3. Austria
Austrian Agency for Health and Food Safety (AGES)
4. Belgium
Federal Agency for Medicines and Health Products (AFMPS)
5. Canada
Health Canada / Santé Canada
Regulatory Operations and Enforcement Branch (ROEB)
6. Chinese Taipei
Taiwan Food and Drug Administration (TFDA)
7. Croatia
Agency for Medicinal Products and Medical Devices of Croatia
Agencija za lijekove i medicinske proizvode (HALMED)
8. Cyprus
Pharmaceutical Services (CyPHS)
9. Czech Republic
State Institute for Drug Control
Státní Ústav pro Kontrolu Léčiv (SÚKL)
10. Czech Republic
Institute for State Control of Veterinary Biologicals
and Medicines (ISCVBM)
11. Denmark
Danish Medicines Agency (DKMA)
12. Estonia
State Agency of Medicines (SAM)
13. Finland
Finnish Medicines Agency (FIMEA)
14. France
French National Agency for Medicines and Health Products Safety
Agence nationale de sécurité du médicament et des produits de santé (ANSM)
15. France
Agency for Food, Environmental & Occupational Health Safety
Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail (ANSES)
16. Germany
Federal Ministry of Health *
Bundesministerium für Gesundheit (BMG)
17. Germany
Central Authority of the Laender for Health Protection regarding Medicinal Products and Medical Devices *
Zentralstelle der Länder für Gesundheitsschutz bei Arzneimitteln und Medizinprodukten (ZLG)
18. Greece
Greek National Organisation for Medicines
Εθνικός Οργανισμός Φαρμάκων (EOF)
19. Hong Kong SAR, China
Pharmacy and Poisons Board of Hong Kong (PPBHK)
20. Hungary
National Institute of Pharmacy and Nutrition (NIPN)
21. Iceland
Icelandic Medicines Agency (IMA)
22. Indonesia
National Agency for Drug and Food Control (NADFC)
23. Iran
Iran Food and Drug Administration (IFDA)
24. Ireland
Health Products Regulatory Authority (HPRA)
25. Israel
Institute for Standardization and Control
of Pharmaceuticals (ISCP)
26. Italy
Italian Medicines Agency
Agenzia Italiana del Farmaco (AIFA)
27. Japan
Ministry of Health, Labour and Welfare (MHLW) *
28. Japan
Pharmaceuticals and Medical Devices Agency (PMDA) *
29. Korea (Republic of)
Ministry of Food and Drug Safety (MFDS)
30. Latvia
State Agency of Medicines
Zāļu valsts aģentūra (ZVA)
31. Liechtenstein
Office of Healthcare
Amt für Gesundheit (AG)
32. Lithuania
State Medicines Control Agency (SMCA)
33. Malaysia
National Pharmaceutical Regulatory Agency (NPRA)
34. Malta
Medicines Authority Malta (MAM)
35. Mexico
Federal Commission for the Protection Against Sanitary Risks (COFEPRIS)
36. Netherlands
Health and Youth Care Inspectorate*
Inspectie Gezondheidszorg en Jeugd (IGJ)
37. New Zealand
Medicines and Medical Devices Safety Authority (Medsafe)
38. Norway
Norwegian Medicines Agency (NOMA)
39. Poland
Chief Pharmaceutical Inspectorate (CPI)
40. Portugal
National Authority of Medicines and Health Products, IP
41. Romania
National Agency for Medicines and Medical Devices
(NAMMD)
42. Singapore
Health Sciences Authority (HSA)
43. Slovak Republic
State Institute for Drug Control (SIDC)
44. Slovenia
Agency for Medicinal Products and Medical Devices
Javna agencija Republike Slovenije za zdravila in medicinske pripomočke (JAZMP)
45. South Africa
South African Health Products
Regulatory Authority (SAHPRA)
46. Spain
Spanish Agency of Medicines and Medical Devices*
Agencia Española de Medicamentos y Productos Sanitarios (AEMPS)
47. Sweden
Medical Products Agency (MPA)
48. Switzerland
Swiss Agency for Therapeutic Products (Swissmedic)
49. Thailand
Food and Drug Administration (Thai FDA)
50. Turkey
Turkish Medicines and Medical Devices Agency (TMMDA)
51. Ukraine
State Service of Ukraine on Medicines and Drugs Control (SMDC)
52. United Kingdom
Medicines & Healthcare Products Regulatory Agency (MHRA)
53. United Kingdom
Veterinary Medicines Directorate (VMD)
54. U.S.A
U.S. Food and Drug Administration (US FDA)